Brain Repair and Stroke
Brain Repair and Stroke Recovery
“Brain repair” is a fanciful condition of science fiction novels, B movies and funny Youtube videos. Yet, the limited ability of the brain to recover from injuries or diseases means that people with these conditions face a lifetime of physical disability and social isolation. Understanding cellular and molecular mechanisms of tissue reorganization in stroke, brain trauma or dementia may lead to novel therapies for these untreatable diseases. However, studies of the mechanisms of brain repair are more interesting than just for their clinical relevance—they deal with fundamental philosophies of brain function. For example, many of the principles of tissue reorganization in the injured brain resemble similar processes in the developing brain: the formation of new connections, the assembly or re-assembly of cellular circuits and changes in synaptic plasticity to develop new functions. Does tissue repair after stroke resemble neurodevelopment, or does “regeneration recapitulate development”?
Stroke Recovery
Recovery after stroke resembles the process of learning and memory in the normal brain: does the brain use cellular memory systems in its spontaneous biological recovery? I study the mechanisms of brain repair for their clinical relevance and for, well, the fun of it—to study the neurophilosophy of tissue regeneration.
Cortical astrocytes around a vessel, viral labeling and images from work of Dr. Amy Gleichman
Both serve to build the field of Regenerative Medicine as it applies to the brain. Axonal sprouting is the formation of new connections among brain areas. There is a limited process of axonal sprouting in the tissue adjacent to a stroke site. A major focus in the lab is to identify how an adult neuron, which normally does not engage in axonal sprouting, is triggered by stroke to enter into a growth state and form new connections.
We have used selective cell isolation techniques and transcriptional profiling to determine an “axonal sprouting transcriptome” in stroke, and how this molecular program changes with age and over time after stroke. In this transcriptome we have identified new signaling systems between astrocytes and growing axons (Ephrin-A5/EphA4) and novel stroke-induced brain growth factors (GDF10). We have determined that stroke activates epigenetic signaling systems that in part mediate the formation of new connections among movement-control centers. These studies have identified potential drug targets for a stroke therapy. Ongoing studies in the lab are further developing these directions.
Many of the principles of tissue reorganization in the injured brain resemble similar processes in the developing brain.
Thomas Carmichael, MD, PhD
How A Stroke Stuns
A stroke stuns the adjacent brain. Through several publications we identified this concept, and then determined the molecular systems that lead to the dysfunction in adjacent brain areas. Stroke produces a state of diminished neuronal excitability by increasing tonic GABA signaling. This can be reversed with tonic GABA positive allosteric modulators (at the GABAa alpha 5 and d subunits) and enhanced stroke recovery. Stroke triggers the dissolution of adjacent brain circuits, measured using optogenetic imaging of correlated firing in neuronal networks over time. We have shown in work submitted for publication that two molecular systems that control neuronal excitability are altered in stroke, the transcription factor CREB and the receptor CCR5.
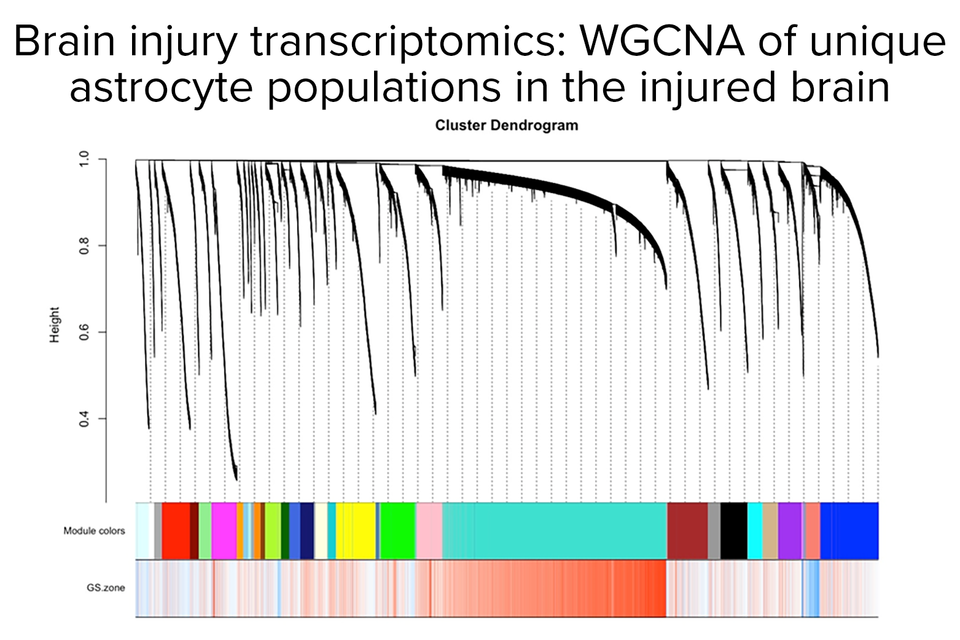
Transcriptomics of astrocytes, Dr. Amy Gleichman
Stimulating these systems enhances stroke recovery by directly altering neuronal circuits in tissue adjacent to the stroke. These three systems, and others that we have published, were first identified as “molecular memory systems”—they enhance normal learning and memory. Our findings in stroke suggest that the brain may co-opt normal ways in which it alters synaptic function to store memories, to modify injured circuits for recovery.
Stroke triggers the brain’s own stem cells to attempt to repair the damage. Stroke induces neural stem cells in the main germinal matrix in the brain, the subventricular zone, to send immature neurons to areas of damage. A small percentage of these cells will survive and mature into new neurons near the stroke site. Stroke also triggers the main progenitor cell in the adult brain, the oligodendrocyte progenitor cell, to proliferate. In both cases of immature neurons and oligodendrocyte progenitor cells, this brain repair response is limited and incomplete. Our work has been some of the first to identify these processes; further goals are now to understand why these stem cell responses in the brain are so limited.
