Why Innovation Lives Here
Collaboration is a Short Walk Away
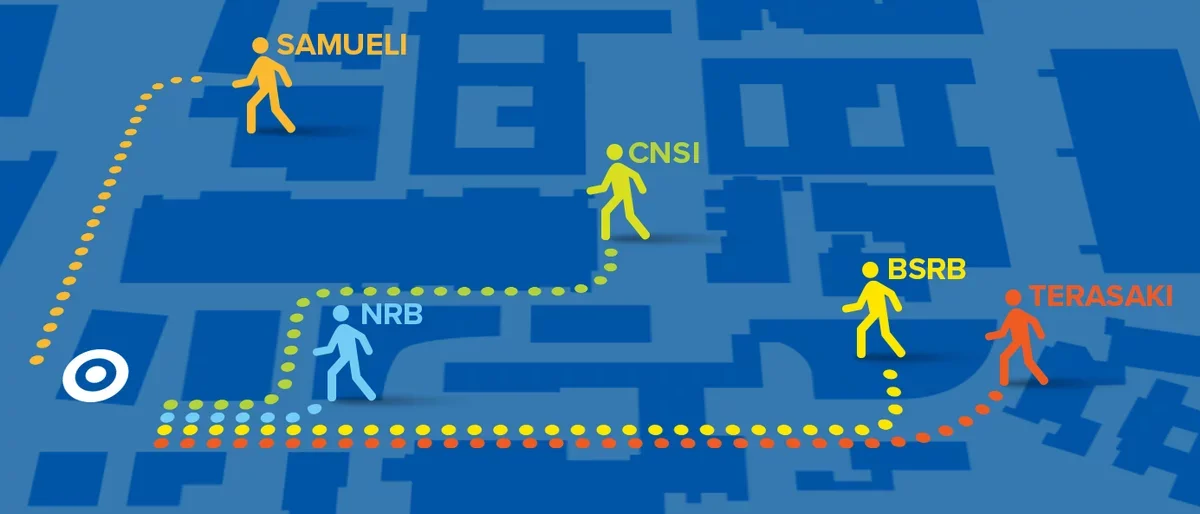
Walk to a range of innovation hubs in less than 5 minutes. UCLA’s compact, walkable campus enables experts across disciplines to share facilities, equipment, and most importantly, conversations that lead to synergistic discoveries.
Innovation Lives Here
It's a 5 minute or less walk from our location to any of the following innovation centers on campus. Your next collaboration is right down the street.
- Terasaki Life Sciences Building
- California Nanosystems Institute
- Biomedical Sciences Research Building
- Henry Samueli School of Engineering
- Neuroscience Research Building
Nearly 75,000 employees and students commute to UCLA on a regular basis.
UCLA Transportation strives to provide smart and sustainable commute options for students, staff and faculty by offering discounted public transit passes, bicycle incentive programs, vanpool programs, carpool parking discounts and more. By embracing a low-car or car-free lifestyle, staff and faculty support UCLA's efforts to create a vibrant, sustainable and healthy campus.
Whether you are on campus or in the surrounding Westwood area, there is plenty to do and explore.
Human Genetics Faculty Research Interests
Our accomplished faculty members collaborate on a wide spectrum of research interests covering everything from the genetic basis of complex traits to computational methods for understanding genetic risk factors for common diseases.
Faculty member
Research Interest
Valerie Arboleda, MD PhD
The overarching research goals in the lab is to integrate large-scale data sets to improve our biological understanding and clinical treatment of human disease.
Paul Boutros, PhD
Personalizing therapy for cancer by developing novel statistical methodologies.
Rita Cantor, PhD
Mathematical modeling of human disease traits.
Stephen Cederbaum, MD
Identification, cloning, regulation and evolution of the two arginases, AI and AII, in man and experimental animals. The goal is to understand the basic biology and pathology of arginine metabolism and then find a treatment for arginase AI deficiency.
Esteban Dell'Angelica, PhD
Genetic Disorders of Organelle Biogenesis and Protein Trafficking.
Eleazar Eskin, PhD
Bioinformatics, Genomics, Computational Methods for Analysis of Complex Traits.
Guoping Fan, PhD
Molecular and cellular mechanisms underlying neuronal differentiation; and generation of mouse models for human diseases.
Jonathan Flint, PhD
Understanding how genes influence behavior, in particular how genetic variation contributes to disease susceptibility.
Brent Fogel, MD, PhD
Basic and fundamental molecular mechanisms underlying human neurodevelopment and neurodegenerative disease.
Nelson Freimer, MD
Genetic basis of complex traits, particularly neurobehavioral disorders including bipolar disorder, schizophrenia, depression, and Tourette Syndrome.
Michael Gandal, MD, PhD
Genetic and genomic sequencing technologies to pinpoint the precise molecular mechanisms in human brain that contribute to neuropsychiatric disorders.
Richard Gatti, MD
Current research focuses on the molecular genetics, diagnostics, radiobiology, and treatment of children with ataxia-telangiectasia, a progressive neurological disease of children.
Dan Geschwind, MD, PhD
The Geschwind laboratory focuses on integrating basic neurobiology, genetics, and genomics with translational studies of human diseases.
Michael Gorin, MD, PhD
Primarily devoted to research and clinical care of hereditary retinal disorders, especially age-related macular degeneration, retinal dystrophies and other medical retinal conditions.
Wayne Grody, MD, PhD
Regulation of gene expression in the human arginase system and arginase deficiency; role of arginase in cancer cell proliferation; technical and ethical aspects of molecular genetic screening; development of novel DNA diagnostics.
Eran Halperin, PhD
Develops statistical and computational methods for the analysis of human genetic and epigenetic variation in the context of complex human diseases.
Deborah Krakow, MD
Define prenatal ultrasound parameters in the osteochondrodysplasias, the molecular basis of musculoskeletal disorders, and the gene expression patterns in the skeleton.
Leonid Kruglyak, PhD
Genetic Basis of Phenotypic Variation.
Kenneth Lange, PhD
Formulation of statistical methods for analyzing the genetic basis of common diseases.
Jingyi Jessica Li, PhD
Statistical methods in biology and genomics, Sparse linear modeling and Bioinformatics.
Kirk Lohmueller, PhD
Population genetics and genomics.
Chongyuan Luo, PhD
Dr. Luo is interested in developing and applying new genomic and genetic technologies to address long-standing questions in human diseases including the causal cell type(s) of diseases and the functions of non-coding genetic variants.
Aldons J. Lusis, PhD
Genetics of complex diseases such as atheroschlerosis using inbred animal strains as models.
Julian A. Martinez-Agosto, MD, PhD
Genetics of growth signaling pathways in stem cells, cancer and overgrowth syndromes.
Stanley Nelson, MD
Technology development for the analysis of complex traits: development of microarrays to study gene expression and SNP scoring.
Genetics of neuropsychiatric traits.
Päivi Pajukanta, MD, PhD
Genetics of cardiovascular diseases.
Christina Palmer, PhD
Centers on genetics of complex behaviors and the educational, psychological, and behavioral outcomes of genetic counseling and genetic testing.
Jeanette Papp, PhD
Bioinformatic solutions for the management and analysis of all types of genetic data.
Bogdan Pasaniuc, PhD
Statistical and computational methods for understanding genetic risk factors for common diseases, particular focus in the study of admixed populations.
Matteo Pellegrini, PhD
Development of computational approaches to interpret genomic data.
Joseph Pisegna, MD
Molecular pharmacology of hormones and receptors in the gastrointestinal tract, diagnosis and management of islet cell tumors of the pancreas.
Karen Reue, PhD
Genes underlying disorders in lipid and glucose metabolism.
Jerome Rotter, MD
Genetics of complex diseases: inflammatory bowel disease, atherosclerosis, diabetes.
Eric Sobel, PhD
Statistical genetics methodologies for large pedigrees.
Sriram Sankararaman, PhD
Novel methods to analyze large-scale genomic data to understand how genetic variation leads to phenotypic variation.
Marc Suchard, MD, PhD
Mathematical and statistical phylogenetics, evolutionary medicine, computational biology, Bayesian methods.
Yi Yin, PhD
Understanding homologous recombination and resulting genome instability.
Stephen Young, MD
During the past few years, we have worked on two general areas, the pathogenesis of hypertriglyceridemia and diseases of the nuclear lamina. Our laboratory works on molecules as they relate to human disease, and we make use of diverse techniques in molecular and cellular biology. When appropriate, we make use of genetically modified mice to investigate our research questions.