Atherosclerosis and Lipid Biology
Atherosclerosis
Atherosclerosis is the single most devastating pathologic process affecting humankind; it accounts for approximately 15 million worldwide deaths every year.
Atherosclerosis occurs when cholesterol and other modified lipids deposit in blood vessels leading to plaque development. The growth of a plaque can lead to sudden rupture or progressive expansion that restricts blood flow to a viral organ. A recent surge in metabolic disturbances, like obesity and diabetes, will ensure that atherosclerosis will not just fade away. Although controlling risk factors like high cholesterol is important, almost half of the hospital admissions for cardiovascular events occur in individuals deemed to be “low risk.”
Why does atherosclerosis affect each individual differently? And how can we improve our prediction as to who will develop significant atherosclerosis? Inspired by these common real-world mysteries, work by Cardiovascular Theme investigators aims to better understand gene-phenotype relationships related to atherosclerosis.
Genes Significant to Atherosclerosis Development

The Sallam lab has discovered that a special group of genes known as long non-coding ribonucleic acids (lncRNAs) play important roles in atherosclerosis development and associated risk factors like dyslipidemia.
What is unique about these genes is that, until recently, they were thought to be “junk” DNA because they do not make a protein product. It is now established that lncRNA genes play important roles and their discovery offers a unique opportunity for the development of novel diagnostic and therapeutic tools in atherothrombotic disease.
We are currently focused on:
- Determining the functional role of human lncRNAs in cholesterol overload states
- Testing the efficacy of lncRNA-based therapeutic strategies in relevant models of human atherosclerosis
- Developing new platforms that will galvanize lncRNA discovery in cardiovascular disease
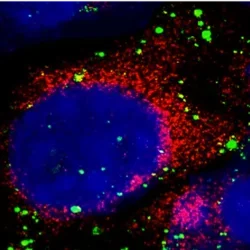
Identifying Novel Proteins
Dr. Stephen Young, Dr. Anne Beigneux and Dr. Loren Fong work together in understanding the basic biology of lipoprotein and triglyceride metabolism and its significance in contributing to vascular and metabolic disease. They have identified a novel GPI-anchored protein (GPIHBP1) that is required for the intravascular processing of triglyceride-rich lipoproteins. Their collaborative efforts have brilliantly demonstrated that GPIHBP1 binds lipoprotein lipase in the interstitial spaces and shuttles it across endothelial cells to its site of action on the capillary lumen. Deficiency of GPIHBP1 results in severe hypertriglyceridemia and their laboratories have identified multiple mutations in humans that result in loss of function of GPIHBP1, inability of the protein to bind lipoprotein lipase and, consequently, severe hypertriglyceridemia.