Cardiac Development and Congenital Heart Disease
Congenital Heart Disease
Congenital heart disease is one of the most common forms of congenital defects and affects nearly 1% of 40,000 live births in the United States annually.
Although major advances have been made in corrective surgeries, the genetic basis of congenital heart defects remains unclear and prognosis in many cardiac abnormalities remain poor. Immense knowledge gaps thus exist in our understanding of the genetic basis and the pathogenesis of progression of congenital heart disease. The Cardiovascular Theme at UCLA, with incredible expertise in cardiac developmental biology and genomics, has made amazing advances in the understanding of congenital heart defects that may lead us to develop therapeutic approaches.
Cardiovascular Developmental Genomics for Congenital Heart Disease

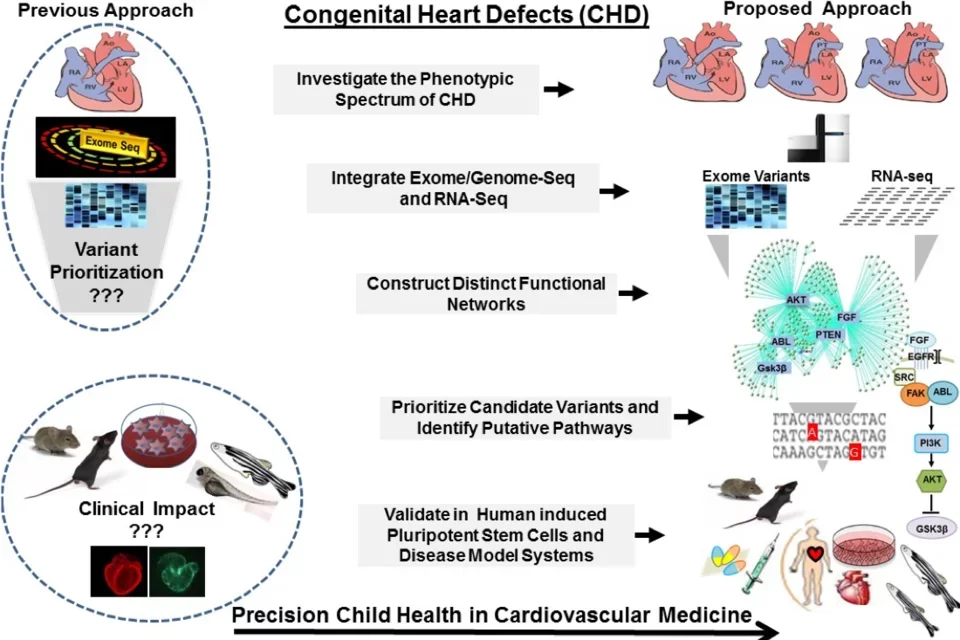
Congenital Heart Defects (CHDs) affect 1% of live births and represent a leading cause of morbidity and mortality in the pediatric population. Contributed by complex genetic, epigenetic, and environmental interactions, the mechanisms of CHDs remain poorly understood. Effective utilization of new discoveries promises to guide strategic approaches toward improved future outcomes for CHDs, but requires narrowing existing gaps between the laboratory bench and the clinic. To address this unmet need, we employ genomics platforms, patient-derived induced pluripotent stem cells, and disease model systems to identify disease-causing genetic variants and to uncover novel gene-environment regulatory circuits that dictate cardiac development and maturation programs and their dysregulation in newborn infants with CHDs. Our unique and well-characterized CHD cohort allows comprehensive and systems-based investigations to elucidate the mechanisms of CHDs, improve the diagnostic yield of genomics platforms, and ultimately develop targeted therapies.
Serving as a central research portal, the UCLA Congenital Heart Defect BioCore synergizes campus-wide clinical efforts and research innovation to integrate the knowledge that we learn from our patients, the resolution of genomics and systems genetic tools, and the depth of functional studies to create a research continuum from genetic discovery to clinical translation, in collaboration with Children's Discovery Institute, the Institute for Precision Health, and the Cardiovascular Theme at UCLA. These endeavors are led by Dr. Marlin Touma, Assistant Professor of Pediatrics, and a multi-disciplinary team of physicians and scientists, including experts in Clinical Cardiology, Genomic Medicine, Bioinformatics, and Molecular and Developmental Biology.
Endothelial Cell Maturation and Patterning by the Epicardium
The laboratory of Dr. Quijada studies the function of the epicardium, the outermost layer of heart. Although developmentally derived from the pro-epicardium, an extracardiac structure, the epicardium plays a critical role in cardiac development as well as in post-natal cardiac regeneration and repair. Dr. Quijada’s laboratory studies the molecular mechanism regulating epicardial development and function of the epicardium. The laboratory also specializes in understanding fetal coronary vascular development and the significance of the epicardium and coronary vasculature in cardiac regeneration.

Fetal coronary vasculature development arises from the integration of endothelial cells (ECs) with supportive mural cells and signals stemming from the epicardium. Synchronized with the development of the coronary vasculature, the epicardium undergoes epithelial-to-mesenchymal transformation (EMT)—a process that regulates epicardial cell migration, differentiation and growth factor secretion. Genetic mouse models with limited epicardial EMT show impaired subepicardial mesenchyme formation in addition to dysfunctional vasculature networks, yet the mechanisms underlying the appearance of this catastrophic phenotype are unknown. Our lab aims to identify the specialized epicardial cells and signaling programs responsible for the distinct patterning of vascular pathfinding cues during coronary vasculature development. To achieve this goal, we utilize cell-lineage tracing models coupled with the investigation of transcriptional programs of epicardial cells and ECs by single-cell RNA sequencing. The epicardium’s role in facilitating arterio-venous specification and patterning is emerging as a novel mechanism to support regenerative-based medicine. Our essential goal is to identify fetal vascular guidance mechanisms that may be harnessed for the development of therapies to treat vascular diseases.
“Coronary vasculature formation coincides with the envelopment of the epicardium over the heart. The epicardium provides paracrine cues supporting the formation of the primitive coronary plexus. Following epithelial-to-mesenchymal transformation (EMT), epicardial cells differentiate into non-myocyte lineages (fibroblasts and vascular mural cells) and provide instructive cues for arterio-venous specification of endothelial cells, a process that is dysregulated upon disruption of epicardial EMT. ”
Restoration of Angiogenic Cues for the Treatment of Ischemic Heart Disease
Coronary artery disease (CAD) is the leading cause of myocardial dysfunction and mortality worldwide. CAD occurs when the constriction and/or blockage of blood vessels restricts oxygen and nutrients to the myocardium, leading to a myocardial infarction (MI). As the adult heart is unable to regenerate following MI, the Nakano Lab aims to identify the cellular and molecular programs required to rebuild coronary arteries and mitigate cardiac damage following an ischemic event. Utilizing knowledge from studies conducted during fetal development, they are investigating vascular cell lineages and paracrine signaling programs in order to promote angiogenesis following MI. Restoration of fetal angiogenic factors in the adult heart is accomplished with in vivo delivery of adeno-associated viral vectors followed by evaluation of de novo coronary vessel formation and cardiac repair in response to acute ischemic injury in the heart.
“The adult heart is unable to regenerate lost myocardium and vascular structures after myocardial infarction (MI). Although the epicardium is reactivated and produces pro-angiogenic cues following MI, this response is inefficient in the prevention of maladaptive cardiac remodeling. Adeno-associated virus (AAV) technology provides a therapeutic strategy to restore the expression of angiogenic factors and stimulate angiogenesis in the heart after ischemic injury.”

The Nakano Lab also examines environmental factors that predispose to the development of heart defects. Maternal diabetes and hyperglycemia are commonly associated with heart defects. The Nakano lab discovered that high glucose impairs the cardiomyocyte maturation by overflowing the pentose phosphate pathway (eLife, 2017). This knowledge is being applied to interventions to the congenital heart disease and heart regeneration. Another key non-genetic cue for cardiogenesis is the biophysical environment. We study how biophysical cues are sensed by atrial and ventricular cardiomyocytes (STAM, 2013, Circ Res, 2014).
Development of the Cardiovascular Vertebrate System

The laboratory of Dr. Jau-Nian Chen in the department of Molecular, Cell and Developmental Biology studies development of the cardiovascular system using zebrafish as a model system.
The heart is the first organ to form during development and the fish provides an opportunity to study development of the organ in the live embryo. Dr. Chen uses classic and genetic screens to identify specific mutants and compounds that can modulate phenotype.
Such approaches used by the Chen lab will ultimately inform basic fundamental questions about cardiac morphogenesis, cellular maturation, and abnormalities of electrical propagation.