Cardiovascular Calcification
Vascular Calcification
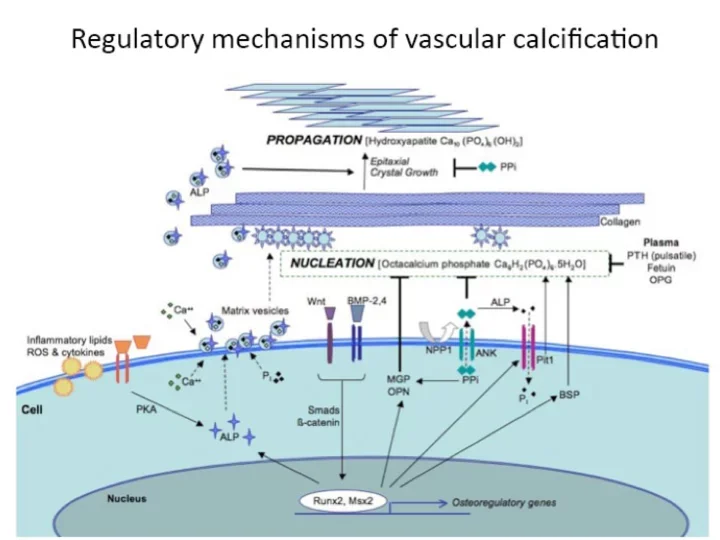
Ectopic calcification is a term used to refer to mineralization of soft tissues outside the skeleton. Ectopic calcification commonly occurs in many highly prevalent diseases such as diabetes, chronic kidney disease, and atherosclerotic heart disease.
Ectopic calcification of the blood vessels is known to lead to dramatic increases in mortality and morbidity. The mineralization of the blood vessels often decreases target organ perfusion and calcification of the walls of the vessel lead to loss of compliance and increased blood pressure. Similarly, calcification of heart valves is an extremely common condition that leads to valvular dysfunction and can only be treated with surgical replacement of the valve. Despite a plethora of clinical literature supporting the adverse outcomes associated with ectopic calcification, no therapies exist for retarding or attenuating ectopic calcification in heart valves or blood vessels. At the UCLA Cardiovascular Research Theme, a combination of vascular biologists and engineers are asking the critical questions in basic and translational biology to usher in potential cures for ectopic calcification.
Consequences of “Hardened” Arteries
UCLA research has helped to identify the process that underlies “hardening” of blood vessels.
It has long been known that bone tissue and even marrow can form in the walls of human arteries, usually near cholesterol deposits.
Previously, says Dr. Linda Demer, Vice Chair of Medicine, artery wall calcification was considered a passive degenerative process. Now, work in Demer’s lab and in others indicates that artery wall cells actively form calcium deposits in much the same way that skeletal bone cells form mineral.

Together with Dr. Yin Tintut, Dr. Demer directs the Cardiovascular Biomineralization Research Group. In collaboration with UCLA engineers, they found:
- The process of calcification in the artery wall is similar to embryonic bone formation.
- There is increased risk of rupture along the edges of calcium deposits facing mechanical stress.
- By isolating and cloning the artery wall cells responsible for producing calcium mineral and by identifying the regulatory molecules controlling the process by which these cells differentiate into bone-like cells, investigators developed the first cell-based model of vascular calcification.
- Based on comparisons to cells from the skeleton, high cholesterol may contribute not only to atherosclerotic calcification, but also to osteoporosis.
Whether calcium deposits protect against, or increase the likelihood of, rupture of coronary plaques—and resulting heart attacks—is a question debated in the cardiovascular disease research community. This research is essential because of the widespread prevalence of vascular calcification, which promotes heart failure and hypertension.
The research program aims to address the adverse effects of coronary calcification and aortic stenosis, with a focus on the mechanism by which calcium mineral forms inside cardiovascular tissues and atherosclerotic plaque. This field began with Dr. Demer’s discovery that the process is regulated at the molecular level and that multipotent vascular stem cells lay down calcium mineral by the same processes driving embryonic skeletal osteogenesis. The lab is characterizing these cells with respect to multilineage capacity, transdifferentiation to osteoblastic cells, and hydroxyapatite nanocrystal formation.
One particularly interesting finding is that, in culture, these cells self-organize into intricate patterns that we can predict and control using computational analysis and reaction-diffusion principles.
Another, relevant to tissue engineering, is left-right chirality—a preference for rightward orientation and alignment when they migrate across micromachined matrix interfaces. The lab’s robust in vitro and mouse models of vascular calcification include quantitative techniques using fused PET-CT imaging, computer-controlled biomechanical testing, atomic force and second-harmonic generation microscopy. The current projects focus on the effects of hyperlipidemia, exercise, and lipid-lowering agents on morphology of vascular calcium deposits and their effects on plaque vulnerability. The findings will provide major clinical implications given the widespread recommendations for use of vitamin D, calcium, exercise, bone-anabolic agents, and cholesterol-lowering drugs.
BMP and MGP in Calcification

The Bostrom Lab’s research interests include vascular calcification, the mechanisms of bone morphogenetic protein (BMP), and the role of the calcification inhibitor matrix Gla protein (MGP) in vascular development and disease.
In addition, the lab is interested in BMP signaling in adipose tissue and mechanisms of cardiomyogenic differentiation in adipose-derived stem cells.
Vascular calcification is a frequent complication in disorders such as atherosclerosis and diabetic vasculopathy. The Bostrom lab has shown that pro-calcific factors can trigger bone-like differentiation in the endothelial cells and in this way promote calcification, and that the BMPs and MGP play important roles in this process.
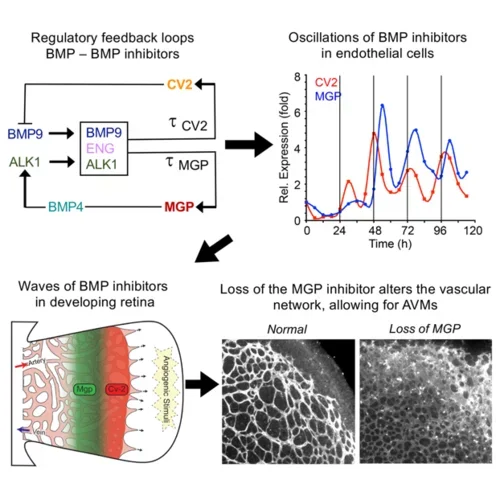
The BMPs are also instrumental in the formation of new vessels. The lab has examined the roles of BMP4 and BMP9 together with their respective inhibitors MGP and Crossveinless-2 (CV2) and found that MGP and CV2 temporally organizes the BMP actions through waves of expression in order to create vascular networks. MGP deficiency disrupts these relationships and causes arteriovenous malformations (AVMs).
The adipose tissue is central in the regulation of metabolism and obesity. The lab is interested in the role of the BMPs and the vasculature in adipogenic differentiation. The adipose tissue is also a well-known source of adult stem cells, and the lab has identified and characterized a population of small stem cells that are able to undergo cardiomyogenic differentiation in the presence of micro-RNA, exosomes, and the leukemia inhibitory factor (LIF).
The goal is to identify new signaling pathways that are activated in cardiovascular disease and regeneration and can be targeted in new treatment strategies.
Arteriovenous Malformations, Vascular Calcification, and Vascular Biology

The laboratory of Dr. Yucheng Yao focuses on several aspects of cardiovascular disease such as cerebral and pulmonary arteriovenous malformations (AVMs) and the prevention of vascular calcification. The lab is also broadly interested in the vascular-related diseases, such as corticosteroid-associated osteoporosis and pulmonary fibrosis. The lab has discovered that ill-fated cell transitions occurring in endothelial cells (ECs) play an essential role in vascular disease and cause the ECs to become a source of pathological cells.
To prevent or reverse this, we are creating novel approaches to shift the ill-fated ECs back to normal differentiation and regain functional capacities, which reverse the course of these disease processes.
Dr Yao’s lab is combining several cutting-edge techniques to achieve the goals. They use molecular tools to examine human specimens and animal models to reveal pathological progression. By examining gene expression profile at single-cell resolution, the lab can uncover the ill-fated differential trajectories in the cells and identify relevant target genes or genetic perturbations. With a high-throughput system, they screen small chemical compounds to target specific genes or signaling pathways. By using high-resolution imaging together with molecular biology approaches, they validate the effects of the compounds in cellular and animal models.
By using these approaches for disease targeting, the studies have successfully identified several compounds with effects on vascular calcification and AVMs. These compounds may be starting points for new therapeutic strategies and clinical translation.