Arrhythymia, Sudden Cardiac-Death, and Ion Channel Biology
Cardiac Arrhythmia
Electrical impulses in a normal heart keep the heart beating. When these impulses are interrupted, the heart can beat too fast, too slow, or with an irregular pattern—all forms of arrhythmia. Arrhythmia contributes to approximately 200,000-300,000 sudden deaths per year—a higher incidence than stroke, lung cancer, or breast cancer.
Electrical Problems With The Heart And Sudden Cardiac Death
A famous athlete collapses and dies during the game. A neighbor is found dead at home but never had any symptoms.
In a moment, a person we knew or loved is gone. It’s known as sudden cardiac death, and it’s frightening. Why does it happen?
Surprisingly, a blocked artery or a heart attack often is not the cause of sudden cardiac death, as most people believe. The problem is usually with the electrical impulses of the heart, and UCLA researchers are studying why it happens and how to prevent it.

Cardiologists can help people live with some forms of arrhythmia, but the abrupt and deadly arrhythmias keep researchers and physicians awake at night. That’s why a team of researchers based at the UCLA Cardiac Arrhythmia Center is focused on:
- Discovering causes of electrical problems with the heart
- Developing treatments and cures for arrhythmia
“If we can correct the issues that cause arrhythmias and find new, less invasive ways to treat them, we will go a long way toward reducing the incidence of sudden cardiac death,” says Dr. Kalyanam Shivkumar, director of the UCLA Cardiac Arrhythmia Center as well as chief of the UCLA Interventional Cardiology Program and the Adult Cardiac Catheterization Laboratory.
Demystifying Scars
Scars on the heart may be caused by a past heart attack or simply by aging. Understanding the functional impact of these factors has revolutionized research in arrhythmias.
The cardiology community previously believed that scars on the heart did not disrupt the electrical flow. They believed electrical impulses would learn to bypass the dead tissue and continue with their normal conduction. Within the past several years, however, research by UCLA and other investigators has found that scars do in fact cause problems for the heart:
- The scars can cause short circuits that trigger life-threatening arrhythmias called ventricular tachycardia or ventricular fibrillation.
- Electricity that flows through a scarred heart is activating the heart in a very abnormal way, according to Olujimi Ajijola, an expert in clinical and interventional electrophysiology at the Cardiac Arrhythmia Center. “The heart will start to beat chaotically, even after one or two beats, which often leads to sudden cardiac death.”
Controlling the Nervous System to Prevent Sudden Death
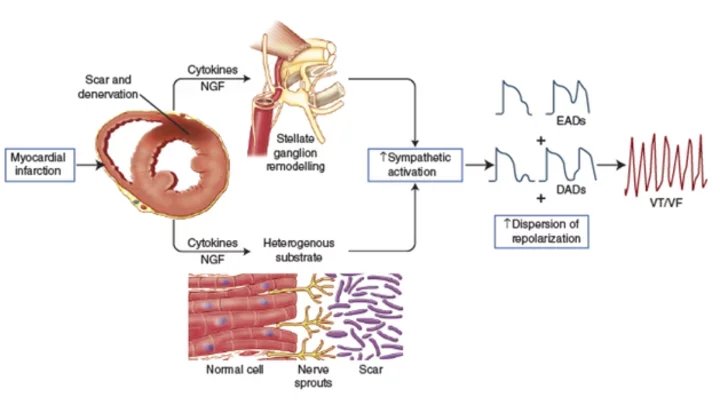
The nervous system influences all aspects of heart function. As Dr. Jeffrey Ardell describes it: “Our minds and our hearts are intimately connected in ways that we are only now discovering.”
Researchers in the medical school have discovered that the nervous system also can play a powerful role in the development of arrhythmias—and their treatment. In fact, UCLA is one of the nation’s preeminent centers for neurocardiology research.
The Nervous System’s Role After Heart Attack
Investigators at the UCLA Neurocardiology Research Center of Excellence, directed by Dr. Ardell, have shown that when the heart is injured, the characteristics of neurons that control the heart also change.
A cover story in the January 2016 issue of the Journal of Physiology detailed how a heart attack alters both the size and the function of neurons that control the heart:

- The brain and the central nervous system sense when the heart is injured.
- The nervous system tries to correct for the injury.
- Over time, these adaptive changes become disease-causing themselves.
Dr. Vaseghi, director of clinical and translational research at the UCLA Cardiac Arrhythmia Center investigates the interplay of the sympathetic and parasympathetic system in contributing to arrhythmias particularly after heart injury and uses murine and porcine models to investigate such questions. Dr. Vaseghi is a principal investigator on an NIH-funded trial to determine the effectiveness of neuromodulation interventions in patients with refractory ventricular arrhythmias.
Ion Channel Biology
Ion channels and their role in cellular excitability are central themes of the research programs developed in the laboratory of Dr. Riccardo Olcese at UCLA, which integrates molecular-level biophysical studies with organ-wide phenomena of clinical significance, such as cardiac arrhythmia. The quantitative tools of biophysics are used in a translational context to understand the role of ion channels in health and disease.

We are investigating the arrhythmogenic properties of anomalies of the repolarization phase of the cardiac action potential known as Early Afterdepolarizations (EAD). We are searching for novel interventions to prevent EADs and their arrhythmogenic consequences by modulating the activity of cardiac L-type calcium channels without adverse effects on contractility (excitation-contraction coupling).
The laboratory also explores the fundamental mechanisms of ion channel function. Ion channels are exquisitely complex membrane proteins that control specific ionic fluxes across the cell membrane controlling cell excitability.
We learn about the molecular and structural basis of ion channel function and regulation in the heart, muscle, and nerves. We are particularly interested in voltage-activated channels, which respond to changes in the membrane potential with a rearrangement of their voltage-sensing structures. We study how their voltage-sensing structures operate driving the channel into ion-conducting (open) and closed conformations. We use state-of-the-art techniques in electrophysiology, biochemistry, molecular biology, and computational modeling. By combining optical methods with electrophysiology, we track in real time the structural changes of ion channel associated with their operation (Voltage-Clamp Fluorometry).
Computational Approach to Cardiac Arrhythmias

A team of investigators in the UCLA Cardiovascular Theme are combining mathematical biology and computer simulation (Drs. Zhilin Qu and Alan Garfinkel) with experimental arrhythmia biology (Riccardo Olcese, Thao Nguyen and Hryar Karagueuzian) to unravel the mechanisms underlying the electrical instability in the heart that causes sudden cardiac death from ventricular fibrillation and complications such as strokes from atrial fibrillation.
They are exploring novel ways to modify the electrical properties of the heart using drugs and gene therapy approaches to prevent the life-threatening complications of these arrhythmias. In modeling of cardiac arrhythmias, Dr. Zhu uses models of stochastic Markovian transitions of ion channels, and ordinary and partial differential equations to simulate subcellular, cellular, tissue, and whole-heart calcium and voltage dynamics that are associated with arrhythmias. They collaborate with experimentalists to test our theories and to reveal the underlying mechanisms of experimental observations.