Mitochondrial Quality
Mitochondrial Decline
Mitochondrial Decline In Quality
Mitochondria are the essential energy engines of the body’s cells. They are the powerhouses at the center of our being. When mitochondria have quality control issues, these problems cause disorders in people’s health. UCLA teams are working to find new ways to understand and support mitochondrial quality.
Mitochondrial Decline & Repair Process
UCLA researchers are fascinated by mitochondria, which are a type of organelle – a tiny, organ-like structure within cells.
Mitochondria use constant decision-making to repair themselves. Van der Bliek describes the process this way:
- Two or three mitochondria fuse together.
- The mitochondria sort the bad components to one end of the organelle.
- The mitochondria divide that segment off.
- The mitochondria ask, “Is that part so bad that we want to get rid of it? Or do we want to re-fuse with the network and continue?”
- If a separated segment is beyond repair, the mitochondria send that segment to be destroyed.
- Mitochondria rejoin and cycle through this quality control process continually.
Collaborators to know
“The clinical goal of this work is to improve these cleaning mechanisms if they are not working properly. That, in principle, would affect a lot of degenerative diseases.”
The Challenge Of Mitochondrial Regulations
Dr. Shirihai has laid out the essential tools that mitochondria use to regulate themselves.
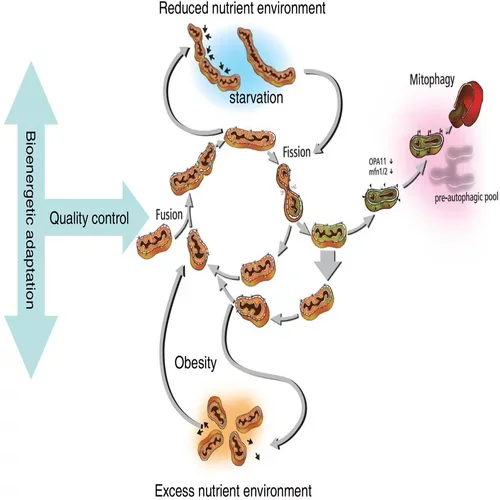
Those essential tools are:
- Fusion: The binding together of mitochondrial bodies
- Fission: The division or coming apart of mitochondria
- Mitophagy: The removal of dysfunctional bits and bodies
Fission and metabolism are connected, as are fission and cell death. Disorders come into play when the process stumbles.
For example, when effective mitophagy declines, it causes a risk factor in multiple diseases, especially neurodegeneration. When non-functioning mitochondria aren’t removed, cells suffer from a lack of fuel availability.
Mitochondrial Damage
Regulation And Disease
UCLA researchers have identified molecular “knobs” that, when mutated, impair the ability of mitochondria to die if defective. These include:
- Cancer: The switch to send an entire cell into apoptosis (programmed cell death) is at the point of mitochondrial fission. Cancer has “learned” to turn that switch off.
- Parkinson’s disease: Another damaged switch leads to development of Parkinson’s disease.
- Alzheimer’s disease: Investigators believe other switches are linked to Alzheimer’s disease and other disorders of aging brains. Read more about research on mitochondria and aging.
Translocon
As Metabolic “Postage System”
Whereas Dr. van der Bliek and Dr. Shirihai study how mitochondria maintain their interior health through size and dynamics, Carla Koehler, PhD, looks outside the mitochondria at the roughly 1,500 different proteins that mitochondria need to do their work. These proteins are produced within other cell organelles and then transported inside mitochondria, passing through one or both membranes.

Carla Koehler, PhD
The machine-like structures of protein complexes on the outside of mitochondria are known as translocons. Translocons let proteins and other molecules in.
Koehler, a professor of chemistry and biochemistry, says translocons essentially create a “postage system.”
Like sending a letter, the cell’s metabolic system has a mail sorting arrangement that decides where all proteins involved in cellular metabolism should go. Translocons, in turn, respond to metabolic conditions. For example:
- The needs of the cell may dictate that low levels of ATP (adenosine triphosphate, the cell’s fuel) should be generated outside of mitochondria so that new cellular parts can be built.
- Translocons may send many proteins to the mitochondria when nutrients are burned for fast and plentiful energy.
Mitochondrial Disease
Manipulating Translocons To Treat Disease
Koehler’s team has begun to develop ways that translocons can help doctors battle specific diseases. Their work includes:
- Treating PH1: Koehler can manipulate this postage system to treat PH1, a rare and potentially deadly genetic childhood kidney disease. In this disorder, mitochondria import an enzyme instead of sending it to a different organelle inside the cell. An FDA-approved compound blocks transport of the enzyme to the errant mitochondrial “mailbox” and takes it to the right cellular address instead.
- MitoBloCKs: Koehler has identified approximately 100 small molecules, which she calls MitoBloCKs. She and her colleagues are testing MitoBloCKs for their ability to control molecules that percolate into mitochondria. Stopping these molecules could provide a strategy to combat Parkinson’s, Alzheimer’s and other diseases. For example, blocking cancer’s ability to inhibit the apoptosis switch, which van der Bliek investigates, could slow the disease.
- Mitochondrial disease: Koehler is testing why some mitochondria assemble with errors that lead to a variety of mitochondrial diseases. As a rule, such errors are worse when present in the muscles, brain cerebrum or nerves.
Motor neurons: With Dr. Shirihai, who invented various tools that measure the rate of protein intake into mitochondria, Koehler is looking at how the speed of protein influx affects stress levels in primary motor neurons.



