Scientists Discover Key Information about the Function of Mitochondria in Cancer Cells
Investigators say findings could lead to new approaches to cancer treatment
Scientists have long known that mitochondria, the "powerhouses" of cells, play a crucial role in the metabolism and energy production of cancer cells. However, until now, little was known about the relationship between the structural organization of mitochondrial networks and their functional bioenergetic activity at the level of whole tumors.
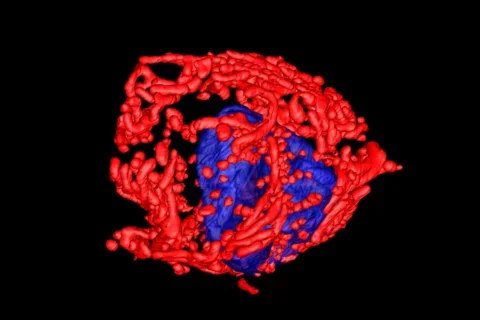
In a new study, published in Nature, researchers from the UCLA Jonsson Comprehensive Cancer Center used positron emission tomography (PET) in combination with electron microscopy to generate 3-dimensional ultra-resolution maps of mitochondrial networks in lung tumors of genetically engineered mice. They categorized the tumors based on mitochondrial activity and other factors using an artificial intelligence technique called deep learning, quantifying the mitochondrial architecture across hundreds of cells and thousands of mitochondria throughout the tumor.
The authors examined two main subtypes of non-small cell lung cancer (NSCLC) — adenocarcinomas and squamous-cell carcinomas and found distinct subpopulations of mitochondrial networks within these tumors. Importantly, they discovered that the mitochondria frequently organize themselves with organelles such as lipid droplets to create unique subcellular structures that support tumor cell metabolism and mitochondrial activity.
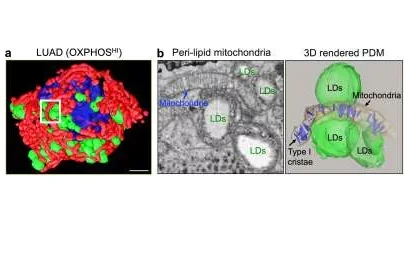
Mitochondria bound to lipid droplets in cells from a subtype of lung cancer. Cells of a type of lung cancer called lung adenocarcinoma, which shows a high level of a metabolic process called oxidative phosphorylation (OXPHOS), contain a subpopulation of mitochondria that are bound to organelles called lipid droplets. a, A 3D reconstruction of a representative lung adenocarcinoma cells with OXPHOSHI signature reveals a compartmentalized mitochondrial (red) population interacting with lipid droplets (green) surrounding the nucleus (blue). Scale bar depicts 3um. b, One peri-droplet mitochondrion can associate with multiple liquid droplets (LDs), and this interaction promotes the organization of infoldings of the inner membrane of mitochondrial (called cristae; blue in the 3D representation in the right image) in parallel. Cristae in this 'type I' form promote high OXPHOS activity, indicating the underlying mechanism for the high OXPHOS signature of these cells. Scale bar depicts 500nm.
The study was led by Mingqi Han, Ph.D., a post-doctoral researcher in the lab of David Shackelford, Ph.D. Dr. Shackelford is a UCLA Jonsson Comprehensive Cancer Center member and Associate Professor of Pulmonary and Critical Care Medicine at the UCLA David Geffen School of Medicine.
The spatial maps of lung tumors that were obtained were rich in data that would take decades to manually annotate by eye. To overcome this bottleneck, the lab partnered with Dr. Stefano Soatto's lab at the UCLA Vision Institute to develop a deep learning neural network that automatically identified and segmented mitochondria networks as well as other cellular organelles within tumors. Image analysis that would have taken over a decade to finish was accomplished in a matter of hours using machine learning software. This work was led by Dr. Han, Dr. Alex Wong and Alexandre Tiard.
The authors anticipate that mitochondrial populations in human cancer samples will not be mutually exclusive to their respective tumor subtype, but rather there will be a spectrum of activity.
The investigators say these findings provide key information about the function of mitochondria in cancer cells and could lead to new approaches to cancer treatment.
“Our study represents a first step towards generating highly detailed 3-dimensional maps of lung tumors using genetically engineered mouse models,” said Dr. Shackelford. “Using these maps, we have begun to create a structural and functional atlas of lung tumors, which has provided us valuable insight into how tumor cells structurally organize their cellular architecture in response to the high metabolic demands of tumor growth. Our findings hold promise to inform and improve current treatment strategies while illuminating new directions from which to target lung cancer.”
"Our study has uncovered a novel finding in the metabolic flux of lung tumors, revealing that their nutrient preference may be determined by the compartmentalization of their mitochondria with other organelles, either relying on glucose (“sugar”) or free fatty acids (“fat”),” said Dr. Han. “This discovery has important implications for developing effective anti-cancer therapies that target tumor-specific nutrient preferences. Our multi-modality imaging approach has enabled us to uncover this previously unknown aspect of cancer metabolism, and we believe that it can be applied to other types of cancer, paving the way for further research in this area."
Article: Han, M. et al. Spatial mapping of mitochondrial networks and bioenergetics in lung cancer. Nature https://doi.org/10.1038/s41586-023-05793-3 (2023).
Original Article: "Scientists Discover Key Information about the Function of Mitochondria in Cancer Cells"